Structure of 1-Propanol Aqueous Solution through Kirkwood-Buff Integrals and Fluctuations
Tetsuo NAKAGAWA
Department of Science Education, Faculty of Education, Gunma University; Aramaki, Maebashi-shi 371-8510 Japan
In order to obtain the basic information of the structure of 1-propanol aqueous solution, the Kirkwood-Buff (KB) integrals, G11, G22 and G12 (1: 1-propanol, 2: water), the concentration fluctuation of 1-propanol, N <(Δx1)2>, the density fluctuation, <(ΔN)2>/N, their correlation term, <ΔNΔx1>, the density fluctuations of respective components <(ΔNi)2>/<Ni> , and their correlation term <ΔNiΔNj>/<Nj> have been estimated over the whole concentration range and at the temperature range of 283.15-- 353.15 K. The KB integrals have been calculated from the thermodynamic properties such as isothermal compressibilities, kT, excess molar Gibbs energies, GmE, and excess molar volumes, VmE. Successively, these KB integrals have been converted to the fluctuations using Nishikawa procedure. In G11, G22 N<(Δx1)2>,<(ΔN)2>/N and <(ΔNi)2>/<Ni>, the maxima have appeared at x1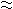 0.2, and increased with the increase of temperature. On the other hands, in G12, <ΔNΔx1> and <ΔNiΔNj>/<Nj>, the minima have appeared at the same mole fraction, and decreased. However, this tendency is reversed in x1 > 0.3. These results suggest us the followings: The self-associations of 1-propanol and water molecules are more preferential in x1 < 0.3 and consequently the microscopic demixing of this system more advances as the temperature increases, in contrast, the hetero-associations between 1-propanol and water molecules are more progressed in x1 > 0.3.
0.2, and increased with the increase of temperature. On the other hands, in G12, <ΔNΔx1> and <ΔNiΔNj>/<Nj>, the minima have appeared at the same mole fraction, and decreased. However, this tendency is reversed in x1 > 0.3. These results suggest us the followings: The self-associations of 1-propanol and water molecules are more preferential in x1 < 0.3 and consequently the microscopic demixing of this system more advances as the temperature increases, in contrast, the hetero-associations between 1-propanol and water molecules are more progressed in x1 > 0.3.
[Contents (In Japanese) ]
[Contents (In English) ]
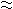 0.2, and increased with the increase of temperature. On the other hands, in G12, <ΔNΔx1> and <ΔNiΔNj>/<Nj>, the minima have appeared at the same mole fraction, and decreased. However, this tendency is reversed in x1 > 0.3. These results suggest us the followings: The self-associations of 1-propanol and water molecules are more preferential in x1 < 0.3 and consequently the microscopic demixing of this system more advances as the temperature increases, in contrast, the hetero-associations between 1-propanol and water molecules are more progressed in x1 > 0.3.
0.2, and increased with the increase of temperature. On the other hands, in G12, <ΔNΔx1> and <ΔNiΔNj>/<Nj>, the minima have appeared at the same mole fraction, and decreased. However, this tendency is reversed in x1 > 0.3. These results suggest us the followings: The self-associations of 1-propanol and water molecules are more preferential in x1 < 0.3 and consequently the microscopic demixing of this system more advances as the temperature increases, in contrast, the hetero-associations between 1-propanol and water molecules are more progressed in x1 > 0.3.