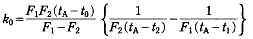
where F1 and F2 are fractions melted corresponding to the temperatures t1 and t2; t0 is the temperature at the end of melting of the mixture, i.e. the liquidus temperature, which is equal to the temperature at F= 1 and tA is the melting point of the pure component, respectively. The solid composition (Cs) equilibrium with the liquid phase at the temperature t0 is given by the product of the initial solute concentration (C0) and k0 obtained by the above equation, i.e. Cs=k0C0. From the result of DSC for the samples containing about 0.5, 1.0 and 2.0 mass% of p-dibromobenzene in p-diiodobenzene and of p-diiodobenzene in p-dibromobenzene, the solidus and liquidus curves on both sides of the low concentrations in the phase diagram were estimated.
[Contents (In Japanese) ] [Contents (In English) ]