Sorption and Separation of Metallic Ions by a Macromolecular Resin Impregnated with 2-Ethylhexyl Hydrogen-2-ethylhexylphosphonate
Masahiro TAKAHASHI*, Yasuhiro YAMASHITA† and Satoru KOSAKA††
Department of Chemistry and Biochemistry, Suzuka National College of Technology;
Shiroko-cho, Suzuka-shi 510-0294 Japan
† Toyohashi University of Technology; 1-1 Hibarigaoka, Tenpaku-cho, Toyohasi-shi
441-8122 Japan
†† Toyota Central Research and Development Laboratories, Inc.; 41-1 Nagakute Yokomichi,
Nagakute-cho, Aichi-gun, Aichi 480-1131 Japan
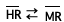 n + nH+
n + nH+
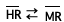 n + nH+
n + nH+